Understanding Gene Functions: An Overview of Gene Ontology and KEGG Pathway Analysis
Gene Ontology (GO) and KEGG Pathway analysis are essential bioinformatics tools for understanding gene functions and their roles within biological systems. GO provides a structured framework for classifying gene functions across different species, organizing them into three main categories: Biological Process, Molecular Function, and Cellular Component. This classification helps researchers comprehend the biological context and interactions of genes. On the other hand, KEGG Pathway analysis maps genes to known biological pathways, allowing scientists to visualize and interpret complex biochemical processes and molecular interactions within the cell. Together, these tools facilitate a deeper understanding of gene functions and their contributions to various biological phenomena. This protocol was developed using a system equipped with an Intel 10th generation i9-10910 processor and 48GB of memory. The test environment includes R version 4.4.0 running on macOS 14.4.1.
Currently, GO/KEGG analysis is generally categorized into web-based and terminal-based formats. In this section, I will elaborate on the functional analysis of genes using the widely-used web-based tool, DAVID, as well as the terminal-based tools, enrichGO and enrichKEGG, which are part of the clusterProfile R package.
Web-based Tool: DAVID
DAVID (Database for Annotation, Visualization, and Integrated Discovery) is a comprehensive web-based tool that offers functional annotation tools for interpreting the biological significance of large gene lists. Integrates multiple annotation resources, including GO and KEGG, for functional annotation, gene ontology enrichment, pathway mapping, and visualization.
| Advantage | Disadvantage |
|---|---|
| - User-friendly interface with integrated resources. - Allows easy upload of gene lists and quick retrieval of functional annotations. - Provides a variety of visualization tools to aid interpretation. | - Limited customization options compared to command-line tools. - Dependent on internet access and server availability. - Can be slower with very large datasets. |
Step-by-Step DAVID Guide
- Go to the DAVID website.
- Click Start Analysis on the website.
- Paste your gene list in the step 1 box.
- In this example, I selected 1962 upragulated DEGs included by osteogenic stimuli and uploaded them. These genes designated as Ensembl IDs rather than gene symbols.
- Select identifier.
- Choose your gene type. In this example, I selected “ENSEMBL_GENE_ID”.
- Note that Ensembl IDs have different prefixes for different species, so specifying a species is not necessary, but some identifiers may require it.
- Select the list type as either gene list or ‘background’.
- Gene List: This is the primary list of genes you are interested in analyzing. It is the focus of your enrichment analysis.
- Background (Optional): This is an optional list used to define the background set of genes against which your gene list is compared. If not provided, DAVID uses a default background set based on the genome of the organism.
- Click Submit List.
- Check the Gene List Manager in the left panel.
- The number of genes that DAVID recognized and those it did not (Unknown) will appear. If there are many unknown genes, consider re-uploading using a conversion tool or another identifier.
- Click Functional Annotation Tool in the center panel.
- DAVID will display the Annotation Summary Results.
- Use the Gene Ontology section and the Pathway section in the results to explore the functional annotation and enrichment of your gene list.
- All data is available for download as tab-delimited text.
Understanding Gene Ontology section
In DAVID functional annotation results, when you click on the GO section, you encounter several subsections labeled as BP (Biological Process), MF (Molecular Function), CC (Cellular Component), followed by labels such as _1 to _5, ALL, and DIRECT. Here is what each of these terms signifies:
- BP (Biological Process): This section pertains to biological objectives or roles completed by sets of molecular activities. Examples include cellular processes and metabolic functions.
- MF (Molecular Function): This section describes the biochemical activity of genes, such as binding or catalysis, independent of the gene products (e.g., protein) performing them.
- CC (Cellular Component): This part describes where gene products are located in the cell, such as membranes or organelles.
- ALL: This category provides a consolidated view of the results across all three GO domains (BP, MF, CC), offering a comprehensive overview without the segregation into specific domains.
- DIRECT: This indicates the annotation derived directly from the primary literature and not inferred from other sources or computational predictions. It is typically more specific and can be considered more reliable as it is directly tied to experimental evidence.
- Lower numbers (like
_1or_2) generally indicate groups of GO terms that are more general and broadly applicable to a variety of biological contexts. As the numbers increase (towards_3,_4, and_5), the GO terms become more specific and detailed. These higher numbers represent more niche and precise annotations that are closely related to the specific functions or locations of the genes in your list.
Understanding GO/KEGG Analysis Output
- Open GO result with R:
> GO_DAVID <- read.table("Up_GO.txt", sep = "\t", header = TRUE) > GO_DAVID Category Term Count Percent PValue Genes ListTotal PopHits 1 GOTERM_BP_DIRECT GO:0006629~lipid metabolic process 119 6.0994362 6.263319e-12 ENSMUSG00000025350 1695 741 2 GOTERM_BP_DIRECT GO:0001666~response to hypoxia 43 2.2039979 1.888764e-07 ENSMUSG00000034205 1695 215 3 GOTERM_BP_DIRECT GO:0006811~ion transport 84 4.3054844 5.390971e-06 ENSMUSG00000031075 1695 605 4 GOTERM_BP_DIRECT GO:0006956~complement activation 12 0.6150692 7.838427e-06 ENSMUSG00000022018 1695 28 5 GOTERM_BP_DIRECT GO:0001649~osteoblast differentiation 25 1.2813942 1.118882e-05 ENSMUSG00000008136 1695 110 PopTotal FoldEnrichment Bonferroni Benjamini FDR 1 20199 1.913766 3.367162e-08 3.367160e-08 3.352128e-08 2 20199 2.383363 1.014884e-03 5.076997e-04 5.054332e-04 3 20199 1.654566 2.856599e-02 9.660621e-03 9.617493e-03 4 20199 5.107206 4.126402e-02 9.976222e-03 9.931685e-03 5 20199 2.708367 5.837805e-02 9.976222e-03 9.931685e-03 - Open KEGG result with R:
> KEGG_DAVID <- read.table("Up_KEGG.txt", sep = "\t", header = TRUE) > KEGG_DAVID Category Term Count Percent PValue 1 KEGG_PATHWAY mmu01100:Metabolic pathways 214 10.968734 3.927550e-11 2 KEGG_PATHWAY mmu00982:Drug metabolism - cytochrome P450 21 1.076371 1.615860e-06 3 KEGG_PATHWAY mmu00760:Nicotinate and nicotinamide metabolism 13 0.666325 1.704787e-04 4 KEGG_PATHWAY mmu05208:Chemical carcinogenesis - reactive oxygen species 37 1.896463 2.304140e-04 5 KEGG_PATHWAY mmu04014:Ras signaling pathway 36 1.845208 1.168008e-03 Genes ListTotal PopHits PopTotal FoldEnrichment Bonferroni Benjamini FDR 1 ENSMUSG00000031231827 1625 9463 9463 1.506898 1.327511e-08 1.327512e-08 1.284309e-08 2 ENSMUSG00000074183 827 71 9463 3.384420 5.460121e-04 2.730804e-04 2.641932e-04 3 ENSMUSG00000115338 827 42 9463 3.541746 5.599775e-02 1.920727e-02 1.858218e-02 4 ENSMUSG00000074183 827 224 9463 1.890066 7.493281e-02 1.946998e-02 1.883635e-02 5 ENSMUSG00000031453 827 235 9463 1.752903 3.263318e-01 6.597445e-02 6.382735e-02
When interpreting the results of a GO or KEGG analysis, several key metrics are presented:
- Count: This metric indicates the number of genes associated with a particular GO and KEGG term within the list of genes being analyzed.
- List Total: Represents the total number of genes analyzed. This value serves as the denominator for calculating proportions of genes associated with specific GO/KEGG terms.
- Pop Hits: Refers to the number of genes associated with the same GO term in the reference gene set, which typically includes all genes in the organism’s genome.
- Pop Total: The total number of genes in the reference gene set. This number is used to normalize the data and helps in calculating how representative the term is in the context of the entire genome.
- Fold Enrichment: This is a crucial statistic that indicates how much more often genes associated with a particular GO term are observed in the list of analyzed genes compared to what would be expected based on their representation in the genome.
Alternative Web-based Tool: Gene Ontology Resource
The Gene Ontology Resource provides web-based tools for exploring GO annotations and performing enrichment analysis. This tools offers access to extensive GO annotations, tools for functional profiling, and interfaces for browsing and searching GO terms. Go to the Gene Ontology Resource.
| Advantage | Disadvantage |
|---|---|
| - User-friendly and accessible without needing programming skills. - Comprehensive and detailed GO term information. - Regularly updated with the latest GO terms and annotations. | - Limited to GO analysis without extensive pathway mapping like KEGG. - Customization and advanced analysis options are less flexible than in command-line tools. - Relies on internet access and web interface performance. |
Depicting a Bubble Chart from GO/KEGG Analysis Results
A bubble chart is an exceptionally effective visual tool for presenting the results of GO and KEGG analyses. This type of chart enhances the visualization of the significance and impact of gene sets across different biological processes, molecular functions, and cellular components. In a bubble chart:
- Each bubble represents a specific GO/KEGG term, providing a direct visual correlation to the biological aspect being analyzed.
- The size of the bubble is determined by the number of genes associated with each GO/KEGG term, visually emphasizing terms with a greater gene count.
- The color of each bubble varies according to the statistical significance (e.g., p-value), offering an immediate sense of the term relevance and the robustness of the associated data.
In this section, I will construct a bubble chart using ggplot2 to display the GO analysis results. The y-axis will list the GO terms, while the x-axis will represent the gene ratio, indicating the proportion of genes linked to each GO term relative to the total number of genes analyzed. The size of each bubble will be proportional to the gene count associated with each term, and the color of bubble will reflect the statistical significance, providing a comprehensive and intuitive visual summary of the data.
Required Packages:
library(ggplot2)
library(dplyr)
library(gridExtra)
library(egg)
library(readxl)
Step-by-Step Guide for Depicting Bubble Chart with Multiple Facets
1. Data Preparation
- Import the Gene Ontology results from text files into R, ensuring that the files have headers in the first row and do not contain row names. This example uses data from a GO analysis of genes upregulated and downregulated by differentiation stimuli in normal mesenchymal stem cells:
UP <- read.table("upDEG.txt", sep = "\t", header = TRUE) DOWN <- read.table("downDEG.txt", sep = "\t", header = TRUE) - Next, select the five GO terms with the lowest False Discovery Rate (FDR).
top_up <- UP %>% arrange(FDR) %>% slice_head(n = 5) %>% mutate(Type = "Up-regulated") top_down <- DOWN %>% arrange(FDR) %>% slice_head(n = 5) %>% mutate(Type = "Down-regulated")
Selecting the top 5-10 terms based on FDR is a common practice but not a strict rule. Researchers are encouraged to tailor the selection criteria based on the specific narrative and needs of their study.
- Combine the top GO terms from both datasets into a single object and compute the
RatioofCounttoList.Totalfor each term:combined <- bind_rows(top_up, top_down) data <- combined %>% mutate(Ratio = Count / List.Total) - Output:
> data Category Term Count X. PValue List.Total Pop.Hits Pop.Total 1 GOTERM_BP_DIRECT GO:0006629~lipid metabolic process 119 6.0994362 6.263319e-12 1695 741 20199 2 GOTERM_BP_DIRECT GO:0001666~response to hypoxia 43 2.2039979 1.888764e-07 1695 215 20199 3 GOTERM_BP_DIRECT GO:0006811~ion transport 84 4.3054844 5.390971e-06 1695 605 20199 4 GOTERM_BP_DIRECT GO:0006956~complement activation 12 0.6150692 7.838427e-06 1695 28 20199 5 GOTERM_BP_DIRECT GO:0001649~osteoblast differentiation 25 1.2813942 1.118882e-05 1695 110 20199 6 GOTERM_BP_DIRECT GO:0007049~cell cycle 190 9.8752599 9.081317e-52 1798 649 20199 7 GOTERM_BP_DIRECT GO:0051301~cell division 126 6.5488565 2.885314e-37 1798 404 20199 8 GOTERM_BP_DIRECT GO:0007059~chromosome segregation 55 2.8586279 9.352744e-28 1798 111 20199 9 GOTERM_BP_DIRECT GO:0006260~DNA replication 55 2.8586279 7.029347e-26 1798 119 20199 10 GOTERM_BP_DIRECT GO:0006281~DNA repair 100 5.1975052 5.008471e-22 1798 395 20199 Fold.Enrichment Bonferroni Benjamini FDR Type Ratio 1 1.913766 3.367162e-08 3.367160e-08 3.352128e-08 Up-regulated 0.070206490 2 2.383363 1.014884e-03 5.076997e-04 5.054332e-04 Up-regulated 0.025368732 3 1.654566 2.856599e-02 9.660621e-03 9.617493e-03 Up-regulated 0.049557522 4 5.107206 4.126402e-02 9.976222e-03 9.931685e-03 Up-regulated 0.007079646 5 2.708367 5.837805e-02 9.976222e-03 9.931685e-03 Up-regulated 0.014749263 6 3.288888 5.278062e-48 5.278062e-48 5.175443e-48 Down-regulated 0.105672970 7 3.503720 1.676944e-33 8.384722e-34 8.221701e-34 Down-regulated 0.070077864 8 5.566470 5.435815e-24 1.811938e-24 1.776710e-24 Down-regulated 0.030589544 9 5.192254 4.085457e-22 1.021364e-22 1.001506e-22 Down-regulated 0.030589544 10 2.844088 2.910923e-18 5.821847e-19 5.708655e-19 Down-regulated 0.055617353In this example, the ‘Genes’ column was excessively lengthy and has been remove to
NULLto streamline the dataset. - When working with data not originating directly from
txtfiles, such as arbitrarily selected GO terms for specific analyses, using Excel can be advantageous. Pre-calculateTypeandRatioin Excel using its functions. This preparation makes the data ready for immediate plotting upon import into R.data <- read_excel("PlotGO.xlsx", sheet = "DOWN_Facet")
2. Plotting
- Draw a Basal Plot:
S1 <- ggplot(data, aes(x = Ratio, y = reorder(Term, Ratio), size = Count, color = PValue)) + geom_point(alpha = 1) + facet_grid(factor(Type, levels = c("Up-regulated", "Down-regulated")) ~ ., scales = "free") + labs(x = "Gene Ratio", y = "") + theme(panel.background = element_rect(fill = 'white', color = 'black', linetype = 'solid', size = 1), panel.grid.major = element_line(color = "grey", size = 0.2), strip.background = element_rect(fill = "grey80", color = "black", size = 1, linetype = 'solid'), strip.text = element_text(color = "black"), legend.key = element_blank(), legend.text = element_text(size = 10), legend.title = element_text(face = "bold"), axis.text.x = element_text(angle = 90, vjust = 0.5, hjust = 1)) + guides(color = guide_colourbar(barwidth = 0.8, barheight = 4)) - Change graphical components:
# Color space S1 = S1 + scale_color_gradient(low = "red2", high = "mediumblue", space = "Lab") # Buble size range can be variable as follow: S2 <- S1+scale_size(range = c(2, 5)) # Adjust the x-axis S3 <- S2 + xlim(0, 0.12) # Adjust chart size for publication, 26.5/25 (width) and 30/33/40 (height) combination S4 <- set_panel_size(S3, width = unit(26.5, "mm"), height = unit(30, "mm")) grid.arrange(S4) - Output:
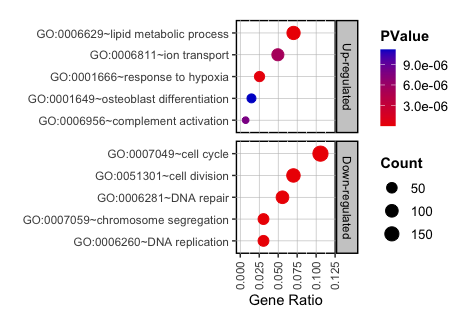
Step-by-Step Guide for Depicting Bubble Chart with a Single Column
1. Data Preparation
- Import the Gene Ontology results from text files into R. This example uses data from a GO analysis of genes upregulated by differentiation stimuli in normal mesenchymal stem cells:
UP <- read.table("upDEG.txt", sep = "\t", header = TRUE) - Next, select the 10 GO terms with the lowest FDR. Once again, selecting 10 GO terms as FDR criteria is not mandatory. Then, calculate the ‘Ratio’ of ‘Count’ to ‘List.Total’.
top_up <- UP %>% arrange(FDR) %>% slice_head(n = 10) data <- top_up %>% mutate(Ratio = Count / List.Total)
2. Plotting
- Draw a Basal Plot:
S1 <- ggplot(data, aes(x = Ratio, y = reorder(Term, Ratio), size = Count, color = PValue)) + geom_point(alpha = 1) + labs(x = "Gene Ratio", y = "") + theme(panel.background = element_rect(fill = 'white', color = 'black', linetype = 'solid', size = 1), panel.grid.major = element_line(color = "grey", size = 0.2), strip.text = element_text(color = "black"), legend.key = element_blank(), legend.text = element_text(size = 10), legend.title = element_text(face = "bold"), axis.text.x = element_text(angle = 90, vjust = 0.5, hjust = 1)) + guides(color = guide_colourbar(barwidth = 0.8, barheight = 4)) - Change graphical components:
# Color space S1 = S1 + scale_color_gradient(low = "red2", high = "mediumblue", space = "Lab") # Buble size range can be variable as follow: S2 <- S1+scale_size(range = c(2, 5)) # Adjust the x-axis S3 <- S2 + xlim(0, 0.09) # Adjust chart size for publication S4 <- set_panel_size(S3, width = unit(26.5, "mm"), height = unit(48, "mm")) grid.arrange(S4) - Output:
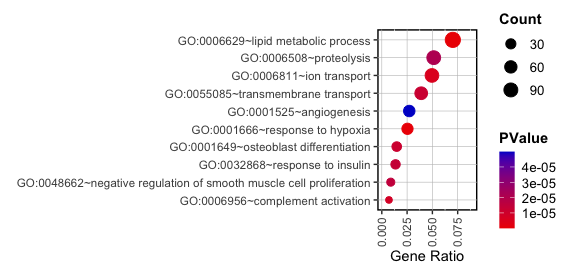
Terminal-based GO Tools: enrichGO and enrichKEGG
The enrichGO and enrichKEGG are powerful R package part of the clusterProfiler suite, designed for the functional annotation and enrichment analysis of gene lists. They provide robust tools for GO and pathway enrichment analyses, allowing researchers to interpret the biological significance of large gene sets through comprehensive statistical analyses.
| Advantage | Disadvantage |
|---|---|
| - High level of customization and flexibility. - Capable of handling large datasets efficiently. - Integrates well with other R packages for further analysis and visualization. - Provides reproducible results as part of R scripts or workflows. | - Requires knowledge of R and command-line operations. - Steeper learning curve for users unfamiliar with programming. - Lack of a graphical user interface, which may be less intuitive for some users. |
Required Packages:
library(clusterProfiler)
library(org.Mm.eg.db) # Organism-specific genome data package, refer to bellow.
library(dplyr)
library(enrichplot)
library(ggplot2)
library(gridExtra)
library(egg)
library(readxl)
Choosing the Appropriate Organism Database
- For organisms other than mouse, you need to use the appropriate organism-specific database. To find the appropriate database for your organism, you can search the Bioconductor website or use the BiocManager package’s search functionality:
BiocManager::available("org.")> BiocManager::available("org.") 'getOption("repos")' replaces Bioconductor standard repositories, see 'help("repositories", package = "BiocManager")' for details. Replacement repositories: CRAN: http://cran.rstudio.com/ [1] "AnnotationForge" "BarBorGradient" "BSgenomeForge" "facebookorganicR" "forge" [6] "IsoCorrectoRGUI" "KTensorGraphs" "org.Ag.eg.db" "org.At.tair.db" "org.Bt.eg.db" [11] "org.Ce.eg.db" "org.Cf.eg.db" "org.Dm.eg.db" "org.Dr.eg.db" "org.EcK12.eg.db" [16] "org.EcSakai.eg.db" "org.Gg.eg.db" "org.Hs.eg.db" "org.Mm.eg.db" "org.Mmu.eg.db" [21] "org.Mxanthus.db" "org.Pf.plasmo.db" "org.Pt.eg.db" "org.Rn.eg.db" "org.Sc.sgd.db" [26] "org.Ss.eg.db" "org.Xl.eg.db" "Organism.dplyr" "OrganismDbi" "organizr" [31] "OrgMassSpecR" "orgR" "orgutils" "OscillatorGenerator" "pd.porgene.1.0.st" [36] "pd.porgene.1.1.st" "PriorGen" "rNeighborGWAS" "RNGforGPD" "sectorgap" - Then you can install the required organism database using Bioconductor:
BiocManager::install("org.*.eg.db") # '*' is organism abb. library(org.*.eg.db)
Step-by-Step enrichGO Guide
1. Data Preparation
Load raw DEG data obtained from
edgeRor user-modified DEG data into R, ensuring that the files have headers in the first row and do not contain row names. In this section, I will load the raw data of gene list (result ofedgeR) that change on osteogenic stimuli in mouse stem cells, filtering out only the DEGs that are upregulated:# Load data data <- read.csv("DEG.csv", header = T) # Filter data UP <- data %>% filter(logFC >= 1, FDR < 0.05)> head(UP) Symbol ensembl_gene_id logFC logCPM PValue FDR osteoMarker 1 Tbx2 ENSMUSG00000000093 3.044420 0.1584658 8.460000e-09 1.020000e-07 2 Axin2 ENSMUSG00000000142 1.695402 -0.9306477 8.412736e-03 2.965543e-02 Osteo 3 Itgb2 ENSMUSG00000000290 2.506077 0.8950346 2.250000e-11 3.870000e-10 4 Fap ENSMUSG00000000392 2.422669 0.4124939 1.695242e-03 7.284350e-03 5 Septin1 ENSMUSG00000000486 1.792568 2.3315912 8.820000e-12 1.600000e-10 6 Acvr1b ENSMUSG00000000532 1.094280 5.3096726 3.170000e-07 2.980000e-06- Next, check the resources of the inputs available to enrichGO.
keytypes(org.Mm.eg.db)> keytypes(org.Mm.eg.db) [1] "ACCNUM" "ALIAS" "ENSEMBL" "ENSEMBLPROT" "ENSEMBLTRANS" "ENTREZID" "ENZYME" "EVIDENCE" [9] "EVIDENCEALL" "GENENAME" "GENETYPE" "GO" "GOALL" "IPI" "MGI" "ONTOLOGY" [17] "ONTOLOGYALL" "PATH" "PFAM" "PMID" "PROSITE" "REFSEQ" "SYMBOL" "UNIPROT" - In this case, I can use the
Symbolrow and theensembl_gene_idrow as input from theUPobject. This corresponds toSYMBOLandENSEMBLin the keytype information (keytypes(org.Mm.eg.db)), which means that enrichGO supports both gene symbol and gene ID row as resource keytypes. For this example, I will useENSEMBLas the keytype.
2. Run enrichGO and enrichKEGG
- Although a filter has been applied, the example output remains extensive due to the substantial number of genes under analysis. Therefore, in this section, I will focus on filtering only the osteogenic markers among the upregulated DEGs:
data <- read.csv("DEG.csv", header = T) Filter_UP <- data %>% filter(logFC >= 1, FDR < 0.05, osteoMarker == "Osteo") - Run
enrichGO:ego <- enrichGO(gene = Filter_UP$ensembl_gene_id, keyType = "ENSEMBL", OrgDb = org.Mm.eg.db, ont = "BP", pAdjustMethod = "fdr", qvalueCutoff = 0.05, readable = TRUE) # Save GO results write.csv(ego, "GOenrich.csv")- in these codes,
gene = Filter_UP$ensembl_gene_id: Specifies the list of genes to analyze. In this case, it uses theensembl_gene_idcolumn from theFilter_UPdataframe.keyType = "ENSEMBL": Defines the type of gene identifier.OrgDb = org.Mm.eg.db: Specifies the gene annotation database.ont = "BP": Specifies the type of GO terms to analyze. Possible terms areBP,MP, andCC.pAdjustMethod = "fdr": Defines the method for p-value adjustment. Possible methods areholm,hochberg,hommel,bonferroni,BH,BY,fdr,none.qvalueCutoff = 0.05: Specifies the cutoff for q-value.readable = TRUE: If set toTRUE, converts gene IDs in the results to a more readable form (e.g., gene symbols).
- Before running
enrichKEGG, check the KEGG organism code:search_kegg_organism('Mus musculus', by='scientific_name') # 'Mus musculus' specifies your organism. - Output:
> search_kegg_organism('Mus musculus', by='scientific_name') kegg_code scientific_name common_name 30 mmu Mus musculus house mouse - Run
enrichKEGG:ekegg <- enrichKEGG(gene = Filter_UP$ensembl_gene_id, keyType = "ENSEMBL", organism = "mmu", # This value (mmu) can be obtained from above 'search_kegg' code. pAdjustMethod = "fdr", qvalueCutoff = 0.05) # Save KEGG results write.csv(ekegg, "KEGGenrich.csv")
3. Visualization
- The
enrichplotpackage offers a variety of visualization methods for the GO and KEGG analyses obtained from theenrichGOandenrichKEGGresults. In addition toenrichplot, bubble charts can be created using theggplot2package by selecting the desired GO/KEGG terms from the saved csv file (refer to the Depicting a Bubble Chart from GO Analysis Results section). In this section, I will present only some of the most frequently used features of enrichplot based on the GO results. - When using
enrichGOandenrichKEGGconsecutively and then visualizing the results, it is not necessary to load the data separately. Adjusting the graphical parameters is beyond the scope of this lesson. For further details, please refer to the enrichplot manual.
3-1. Bar plot:
barplot(ego, showCategory=15)
barplot(ekegg, showCategory=10)
3-2. Bubble chart:
dotplot(ego, showCategory=15)
dotplot(ekegg, showCategory=10)
Output (Left; Barplot, Right; Dotplot): (Clicking on the image will open it in a larger view.)
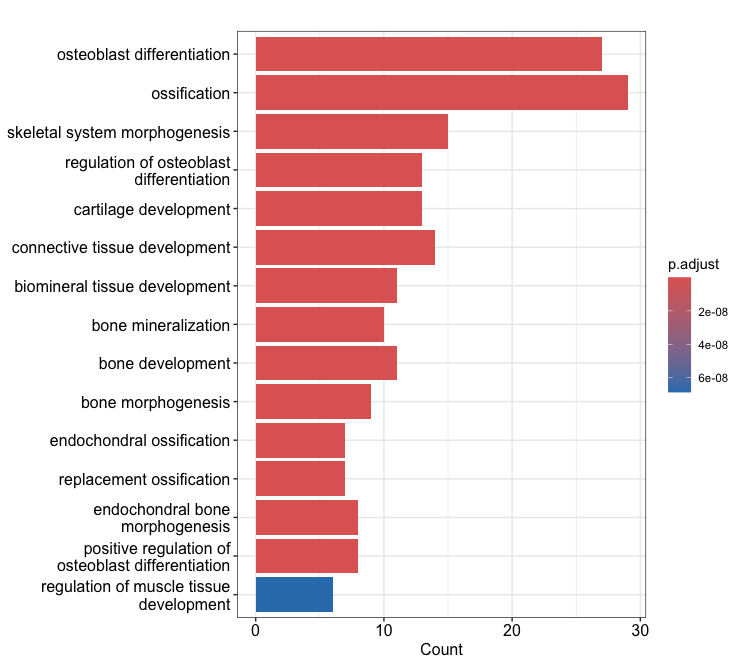
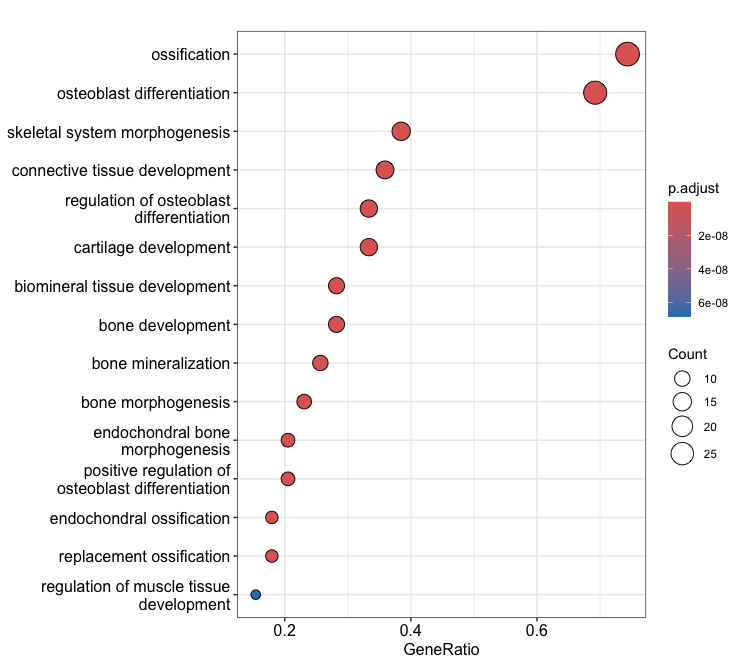 × ❮
× ❮❯
3-3. Gene-concept network:
geneList <- setNames(Filter_UP$logFC, Filter_UP$ensembl_gene_id)
ego2 <- setReadable(ego, "org.Mm.eg.db", "ENSEMBL")
p1 <- cnetplot(ego2, color.params = list(foldChange = geneList), showCategory = 5) +
scale_color_gradient(name = "logFC", low = "blue", high = "red")
p2 <- cnetplot(ego2, color.params = list(foldChange = geneList),
circular = TRUE, colorEdge = TRUE, showCategory = 5) +
scale_color_gradient(name = "logFC", low = "blue", high = "red")
Output: (Clicking on the image will open it in a larger view.)
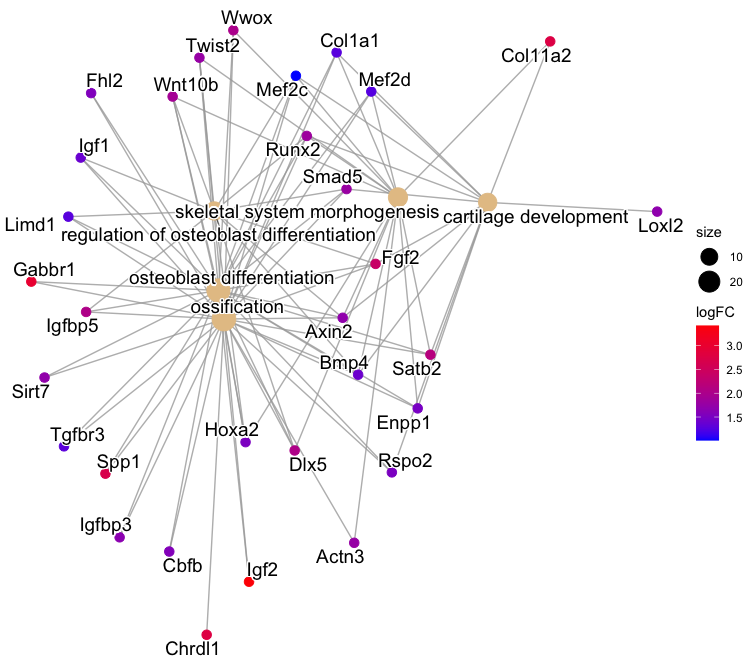
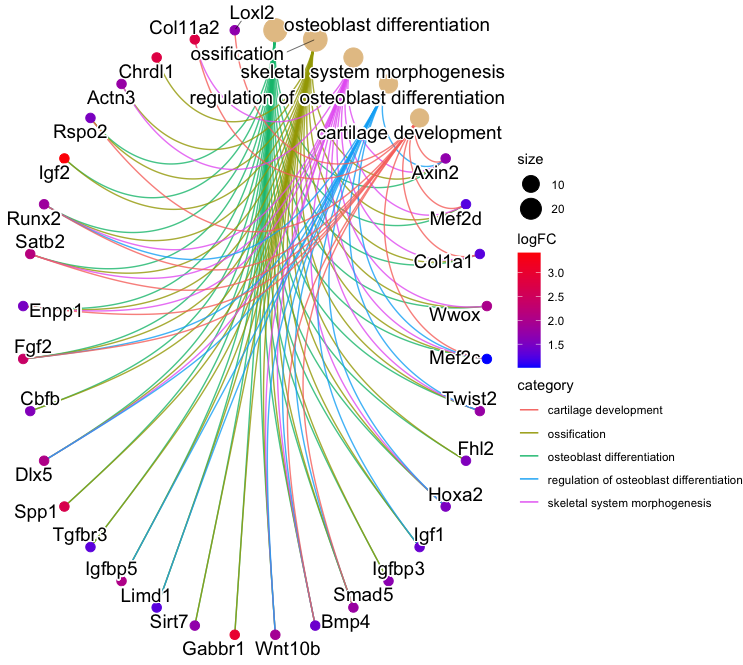 × ❮
× ❮❯
3-4. Heatmap-like functional classification:
p1 <- heatplot(ego2, foldChange = geneList, showCategory = 5)
p2 <- p1 +
scale_fill_gradient(name = "logFC", low = "blue", high = "red") +
coord_fixed(ratio = 1) +
guides(fill = guide_colorbar(barwidth = 0.8, barheight = 5))
- Output:
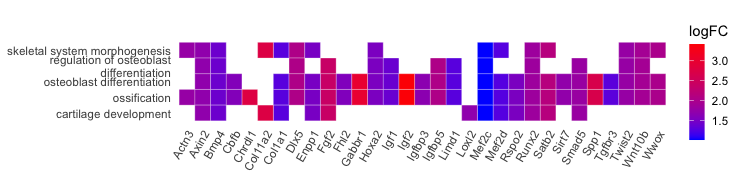
3-5. Tree plot
egoTree <- pairwise_termsim(ego2)
p1 <- treeplot(egoTree, hclust_method = "average")
- Output:
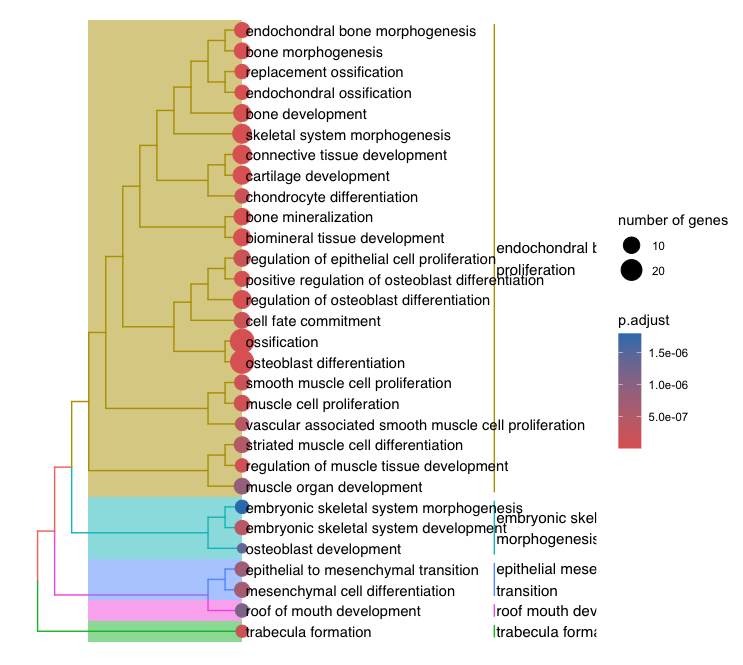
3-6. Gene Set Enrichment Analysis (GSEA)
- GSEA is a computational method that determines if a predefined set of genes shows statistically significant differences between two biological states by comparing gene ranks within the set to those in the entire genome.
- GSEA is crucial in RNA-seq analyses as it reveals differentially regulated biological pathways and processes, offering a comprehensive understanding beyond individual gene expression changes.
- GSEA effectively analyzes the human transcriptome/genome using the Molecular Signatures Database and tools from the Broad Institute. In this section, I will provide code to plot GSEA correlation plot, and create a separate section and explain more about GSEA later.
Data selection:
data <- read.csv("DEG.csv") # Load raw DEG data obtained from edgeR result filtered_data <- data %>% filter(!(logFC > 1 & FDR > 0.05) & !(logFC < -1 & FDR > 0.05))- Output:
> head(filtered_data) Symbol ensembl_gene_id logFC logCPM PValue FDR osteoMarker 1 Gnai3 ENSMUSG00000000001 -1.028899 7.5123714 7.94000e-07 6.96e-06 2 Cdc45 ENSMUSG00000000028 -2.245032 4.7831577 1.61000e-22 8.34e-21 3 Scml2 ENSMUSG00000000037 -1.842400 2.3288016 4.06000e-10 5.96e-09 4 Apoh ENSMUSG00000000049 -0.161434 -0.1108714 8.26452e-01 1.00e+00 5 Narf ENSMUSG00000000056 -1.124729 5.6864476 1.27000e-07 1.28e-06 6 Cav2 ENSMUSG00000000058 -1.028229 6.7405093 1.12000e-06 9.54e-06 Run GSEA:
# Make samll varience (based on normal distribution) to reduce ties in the preranked stats set.seed(42) filtered_data$logFC <- filtered_data$logFC + rnorm(n = nrow(filtered_data), mean = 0, sd = 1e-5) # Make compare list geneList <- setNames(filtered_data$logFC, filtered_data$ensembl_gene_id) geneList <- sort(geneList, decreasing = TRUE) # Run GSEA gsea_results <- gseGO(geneList = geneList, OrgDb = org.Mm.eg.db, ont = "BP", keyType = "ENSEMBL", pAdjustMethod = "fdr", pvalueCutoff = 0.25, eps = 0, verbose = FALSE) # Call GO terms from the GSEA results go_terms <- gsea_results@result[, c("ID", "Description", "NES", "p.adjust", "qvalue")] osteo_terms <- go_terms[grep("osteoblast", go_terms$Description, ignore.case = TRUE), ]- Output:
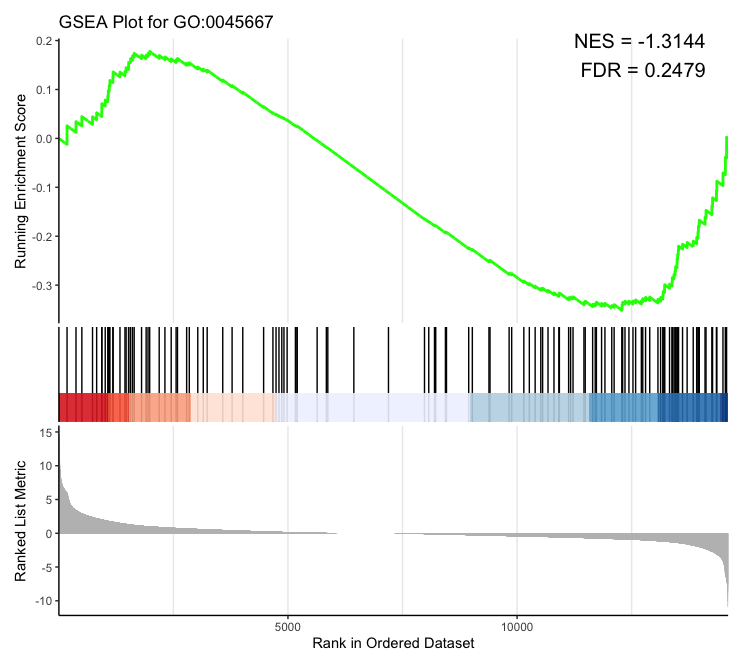
> osteo_terms <- go_terms[grep("osteoblast", go_terms$Description, ignore.case = TRUE), ] > osteo_terms ID Description NES p.adjust qvalue GO:0045667 GO:0045667 regulation of osteoblast differentiation -1.314391 0.2478609 0.2221023 Plotting:
# Make a correlation plot go_id <- "GO:0045667" gseaplot2(gsea_results, geneSetID = go_id, title = paste("GSEA Plot for", go_id)) # Make an information table nes <- gsea_results@result$NES[gsea_results@result$ID == go_id] pvalue <- gsea_results@result$pvalue[gsea_results@result$ID == go_id] padj <- gsea_results@result$p.adjust[gsea_results@result$ID == go_id] lab <- paste0("NES = ", round(nes, 4), "\nFDR = ", round(padj, 4)) ## The 'annotate' function is currently not operational; therefore, I will implement a workaround by overlaying the table on the plot. # Plot with the table grid.text(lab, x = unit(0.95, "npc"), y = unit(0.95, "npc"), just = c("right", "top"), gp = gpar(col = "black", fontsize = 15))- Output:
Citations
- Huang, D. W., Sherman, B. T., Tan, Q., Kir, J., Liu, D., Bryant, D., ... & Lempicki, R. A. (2007). DAVID Bioinformatics Resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic acids research, 35(suppl_2), W169-W175. DOI
- Gene Ontology Consortium. (2001). Creating the gene ontology resource: design and implementation. Genome research, 11(8), 1425-1433. DOI
- Yu, G., Wang, L. G., Han, Y., & He, Q. Y. (2012). clusterProfiler: an R package for comparing biological themes among gene clusters. Omics: a journal of integrative biology, 16(5), 284-287. DOI
- Subramanian, A., Tamayo, P., Mootha, V. K., Mukherjee, S., Ebert, B. L., Gillette, M. A., ... & Mesirov, J. P. (2005). Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences*, 102(43), 15545-15550. DOI</span> </li> </ol> </div>

